1 in 5 breast cancer patients will be impacted by lymphedema1
Lymphedema Prevention Program
Together, We Can End Breast Cancer-Related Lymphedema.

The NCCN Guidelines® are consistent about the importance of early detection of lymphedema and use of an objective tool, such as bioimpedance spectroscopy.
Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Survivorship V.1.2023. © National Comprehensive Cancer Network, Inc. 2023. All rights reserved. Accessed March 24, 2023. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
Reduce the Burden of Breast Cancer-Related Lymphedema (BCRL) through Early Detection Using Bioimpedance Spectroscopy (BIS) and Intervention
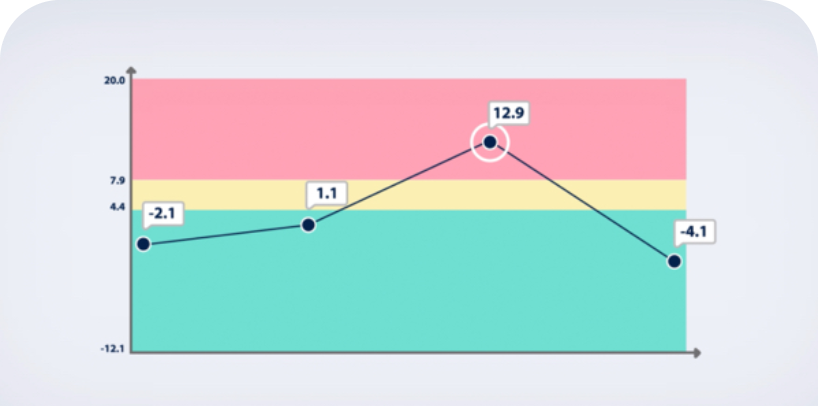
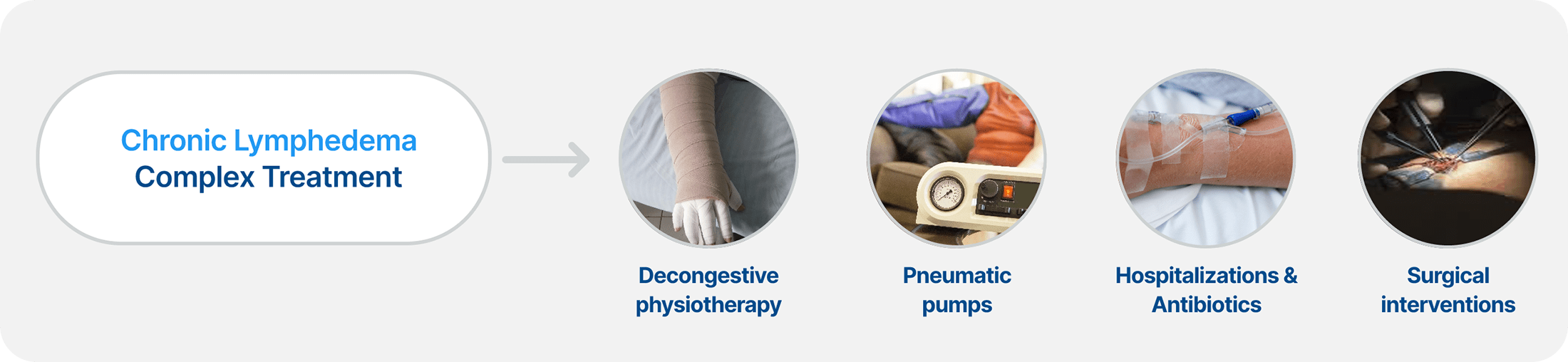
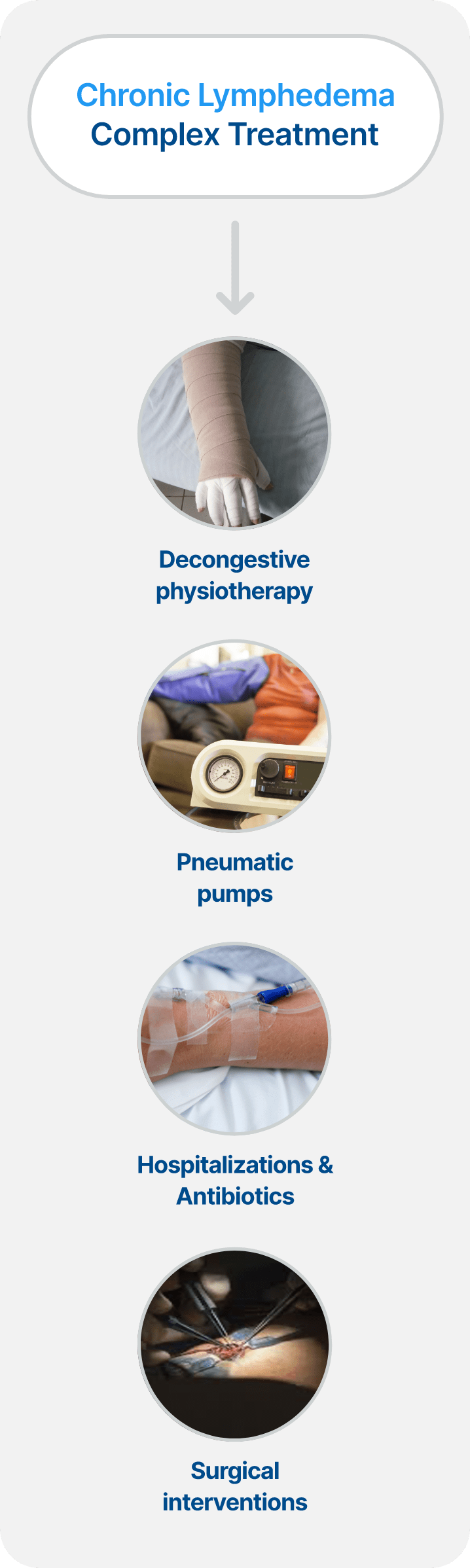
Chronic BCRL Management Can be an Expensive, Complex, and Lifelong Issue for Patients
Chronic BCRL Management Demands Significant Time and Financial Resources
Patients suffering from BCRL often experience a notable decline in their quality of life, and if their disease is left untreated, it can become a life-long condition.
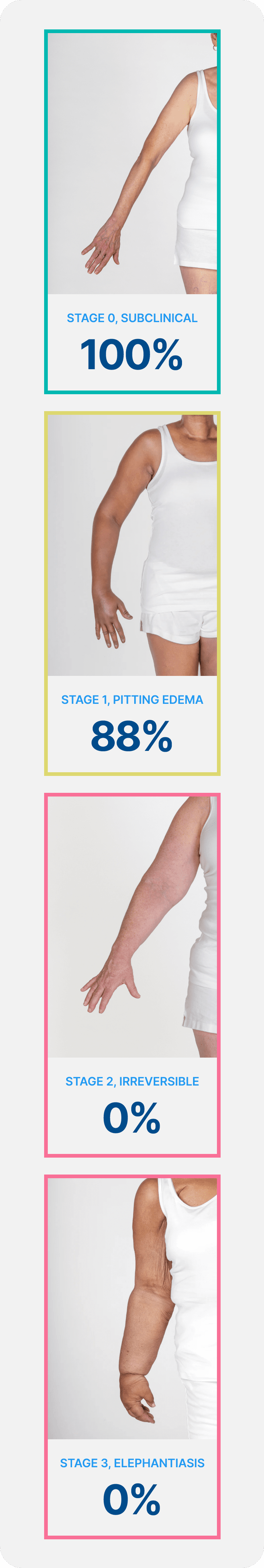
It is Well Established that Breast Cancer-Related Lymphedema (BCRL) can be Resolved with Early Detection and Intervention
Lymphedema develops in stages, and a university of Kansas study found that when lymphedema is detected at stage 0 or stage 1 it is reversible, but in stages 2 and 3 it is not. These findings demonstrate how early conservative intervention and prospective monitoring with BIS can significantly lower rates of BCRL and persistent BCRL (pBCRL).5

Bioimpedance Spectroscopy (BIS) is the Most Effective Method for Lymphedema Detection at the Subclinical Stage
Discover the Benefits of a Lymphedema Prevention Program

Effectively Meet Accreditation Standards for BCRL Care
Successfully align with standards like the National Accreditation Program for Breast Centers (NAPBC) and the Commission on Cancer (COC), which both support lymphedema management throughout cancer treatment.
Streamline Implementation by Leveraging the Expertise of ImpediMed’s Team
Evidence-Based Protocol for Optimal Patient Outcomes
ImpediMed’s Clinical Team has Completed Numerous Successful Implementations Through Innovative Strategies, Multi-Disciplinary Collaboration, and Streamlined Technical Solutions
Innovative Strategies
- Any care team can easily tailor the program workflow to better align with the unique needs of their practice and patients.
- Our lymphedema prevention protocol invites a streamlined approach to patient monitoring and tracking for lymphedema progression.
Multi-Disciplinary Collaboration
- The reimbursement support team is extremely knowledgeable and prepared to work alongside your practice to improve patient access to SOZO testing.
- Our customer support team is equipped with access to valuable resources such as patient education, hospital marketing materials, and training.
Technical Solutions
- ImpediMed’s system and software solutions meet the highest standard of industry and regulatory requirements.
- The care team can easily access SOZO data directly from their EHR system in real-time.
SOZO® Digital Health Platform
With the SOZO Digital Health Platform and L-Dex, ImpediMed is the only company to offer FDA-cleared technology that uses bioimpedance spectroscopy for the clinical assessment of lymphedema.

See how the SOZO Digital Health Platform works.
References
- Gillespie, T. C., Sayegh, H. E., Brunelle, C. L., Daniell, K. M., & Taghian, A. G. (2018). Breast cancer-related lymphedema: risk factors, precautionary measures, and treatments. Gland Surgery, 7(4), 379–403. https://doi.org/10.21037/gs.2017.11.04
- Teo I, et al. Examining pain, body image, and depressive symptoms in patients with lymphedema secondary to breast cancer. Psychooncology. 2015 Nov;24(11):1377-83. Doi:10.1002/pon.3745. Epub 2015 Jan 20. PMID: 25601235.
- Shah C, et al. The impact of monitoring techniques on progression to chronic breast cancer‑related lymphedema: a meta‑analysis comparing bioimpedance spectroscopy versus circumferential measurements. Breast Cancer Research and Treatment 2020; https://doi.org/10.1007/s10549-020-05988-6.
- Dean LT, et al. “It still affects our economic situation.” A long-term economic burden of breast cancer and lymphedema. Supp Care Canc 2017; https://doi.org/10.1007/s00520-018-4418-4.
- Kilgore L, at al. Reducing breast cancer-related lymphedema (BCRL) through prospective surveillance monitoring using bioimpedance spectroscopy (BIS) and patient direction self-interventions. Ann Surg Oncol 2018;http://doi.org/10.1245/s10434-018-6601-8.
- Ridner SH, et al. A Randomized Clinical Trial of Bioimpedance Spectroscopy or Tape Measure Triggered Compression Intervention in Chronic Breast Cancer Lymphedema Prevention. Lymphatic Research & Biology 2022.
- Fu MR, et al. L-dex ratio in detecting breast cancer-related lymphedema: reliability, sensitivity, and specificity. (2013). L-dex ratio in detecting breast cancer-related lymphedema: reliability, sensitivity, and specificity. PubMed. https://pubmed.ncbi.nlm.nih.gov/24354107
- Shah, et al. Bioimpedance Spectroscopy for Breast Cancer Related Lymphedema Assessment: Clinical Practice Guidelines. Breast Cancer Research and Treatment 2022.