About ImpediMed
Founded and headquartered in Brisbane, Australia, ImpediMed is a global company with operations in the United States and Europe. We are the world leader in the design and manufacture of medical devices employing bioimpedance spectroscopy (BIS) technologies for use in the noninvasive clinical assessment and monitoring of fluid status and tissue composition.
Leaders in BIS Technology
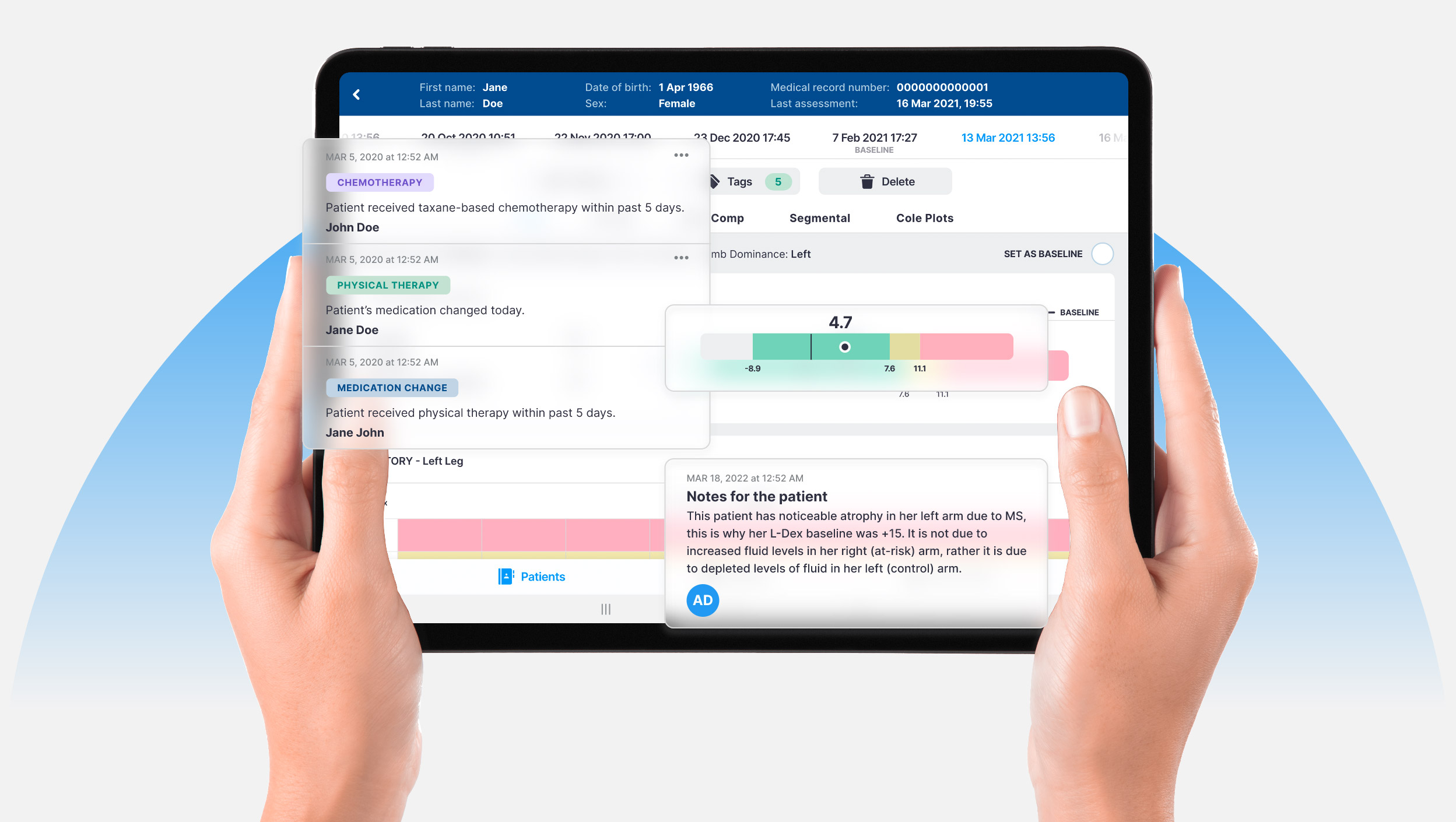
ImpediMed and our subsidiaries pioneered the use of BIS technology, producing the first commercially available BIS devices in 1990. Our L-Dex® device was the first FDA-cleared medical technology to use BIS for the assessment of lymphedema. Use of L-Dex is now recommended in clinical practice guidelines of research centers and professional organizations across the country.

Expanding Applications
In 2017, we launched SOZO®, the world’s most advanced BIS device. The FDA-cleared, CE-marked and ARTG-listed digital health platform aids in the early detection of secondary lymphedema, provides fluid status for patients living with heart failure and allows measurement and tracking of various body composition parameters.
We are currently exploring the use of our advanced bioimpedance technology for a wide range of commercial applications, including:
- General health and weight management
- Bone content
- Protein-calorie malnutrition